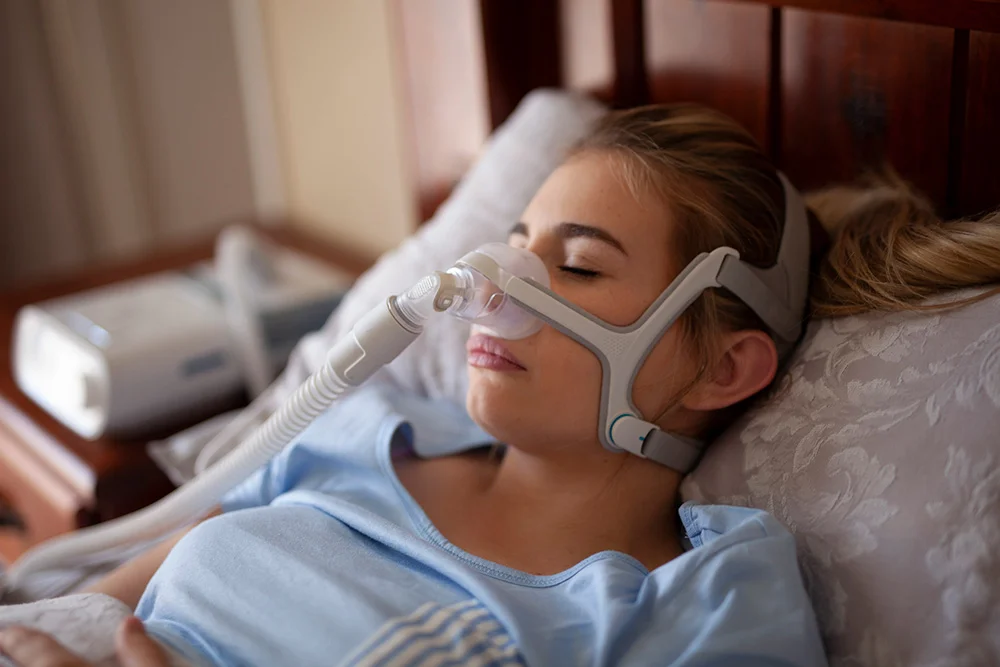
It hasn’t been a great few years for CPAP companies, as a new recall classification for ResMed comes on the heels of numerous other serious recalls for the machines that treat Obstructive Sleep Apnea (OSA). On Jan. 12, the U.S. Food and Drug Administration classified the recall of some specific ResMed respiratory masks as Class 1, the most serious recall classification.
“Use of these devices may cause serious injuries or death,” the FDA decided, typical of Class 1 classifications (1). They included the following devices:
- Product Names: AirFit and AirTouch masks
- Product Codes: See Recall Database Entries:
- Model Numbers: AirFit N10, AirFit F20, AirTouch F20, AirFit N20, AirTouch N20, AirFit F30, AirFit F30i
The recall applies to products that were distributed from January 2020 to November 20, 2023. The recall began in November and includes over 20 million CPAP masks with magnets. On their website, ResMed has also posted more specific guidelines on how and when these types of masks should and shouldn’t be used. These include if the patient or their bed partner or people in close proximity of the mask (such as healthcare workers) have pacemakers, shunts, insulin/infusion pumps, metallic implants such as valves or hearing implants, ocular implants, and other conditions.
To date, six injuries have occurred from the masks. No deaths are documented at this time. The updated protocol is to keep the mask and its magnets at least 6 inches away from any implanted medical device.
The FDA says, “While the existing label advises keeping magnets 2 inches away from affected medical devices, it doesn’t list all the specific ones that could be affected by the masks’ magnets. ResMed is recalling these masks to update the labels, add more warnings and information to guide patients and health care professionals on safe usage when using masks with magnets.”
This isn’t the first company to have a recall for this type of concern with magnets in CPAP masks. In 2022, Philips’ CPAP machines also faced a Class I device recall from the FDA due to similar magnet interference concerns.
Patients should have received information from the company on how to handle the recall on December 8, 2023. If additional information or questions arise, U.S. consumers are encouraged to reach out to ResMed at 1-800-424-0737, the FDA says. They also hope people will reach out to their physicians and suppliers for further information on how the magnets might impact them.
If the road ahead is anything like Philips’ experience, it might be a long and bumpy one to ensure everyone is safe.
Sources
1. U.S. Food and Drug Administration, “ResMed Ltd. Recalls Continuous Positive Airway Pressure (CPAP) Masks with Magnets due to Possible Magnetic Interference with Certain Medical Devices,” https://www.fda.gov/medical-devices/medical-device-recalls/resmed-ltd-recalls-continuous-positive-airway-pressure-cpap-masks-magnets-due-possible-magnetic; January 12, 2024.

How Have the CPAP Recalls Affected Philips’ Finances? So Far, So Good, New Report Shows

Everything You Need to Know About CPAP Devices

FDA Assigns its Most Serious Designation in New Philips CPAP Machine Recall: What to Know